The Cartwright Inquiry (1987 – 1988) was meant to be a watershed moment in patient rights with the establishment of the Code of Rights, and the Health and Disability Commissioner as a champion and protector of these llegally enforceable rights.
But instead, the very existence of the HDC and its applied interpretation of the Code of rights (HDC doctrine), breachs United Nations Universal Declaration of Human Rights:
Article 8: everyone has the right to an effective remedy by the competent national tribunals for acts violating the fundamental rights granted him by the constitution or by law.
Article 5: No one shall be subjected to cruel, inhuman or degrading treatment
Summed up nicely by Peter Skegg, Faculty of Law, University of Otago:
“New Zealand’s experience with a legislated Code of Rights warrants its characterisation as a fortunate experiment”
The Health and Disability Commissioner has a statutory obligation to ‘promote and protect the legal rights of health consumers’. Unfortunately, the notion that the HDC is ‘committed to a consumer centred and engaged system’ is a falsity. Instead, the HDC is acting as an illegal construct, in breach of the United Nations Universal Declaration of Human Rights. Instead of carrying out the intended statutory function through the HDC Code of Rights and acting in the best interests of health consumers, the HDC is protecting healthcare service providers, of which the public health sector is the biggest benefactor.
There is a large body of cases and evidence of fraudulent behaviour by medical practitioners in regards to a number of incidents across the spectrum. There has been an unprecedented amount of patient injuries and deaths that have occurred as the result of malpractice and misrepresentation. (I.e. Synthetic surgical MESH used without patients’ legal consent obtained and as this website exposes with evidence, HDC supplied protection for New Zealand’s largest elected surgical frauds, surgical treatment for gallstones illegally carried out – consent obtained by deception, patents harmed) The HDC have allowed this behaviour to continue under an unwritten ‘you are protected’ application of the Code of Rights. As a direct result, health consumers’ expectations of protection and reasonable access to medical services is sadly misplaced. The ability to receive care and assistance for any harm or wrongdoing is also sadly misplaced.
Expectations rightly expected under normal common law and natural justice include:
- Correction – Where wrongdoing has occurred there should be an independent authority review and assessment of individual practitioner competence, to ensure that similar incidents do not happen to others in the future;
- Accountability/Sanctions – Where there is wrongdoing, practitioners should be held accountable and face professional disciplinary charges, or otherwise be held account for offences under relevant statute law: and
- Restoration –Where there is wrongdoing there should be restorative channels for patients to pursue, including compensation for financial, physical and non-economic loss. Also, there should be restoration to provide for the future care of injured patients.
The Accident Compensation Act has effectively barred civil litigation against a doctor for medical malpractice / misadventure in New Zealand. The only provision for compensation is through the Accident Compensation Corporation – and the only option for a health consumer to process a medical malpractice complaint is through the HDC.
Both of these systems are meant to provide transparency, accountability and redress in situations where the health system has failed. A system, which we are told is ‘the envy of world leaders in the field of patient safety’.
As their role as gatekeeper under the HDC’s application of the Health and Disability Commissioner Act, they have restricted access to The Human Rights Review Tribunal – the only body that can make a damages award upon a finding of breach of the Code. Very few complaints ever make it to the Human Rights Review Tribunal (HRRT), Director of proceedings, the Medical Council of New Zealand, or the Health Practitioners Disciplinary Tribunal for further resolution, or further investigation.
The HDC is reluctant to investigate any complaints against medical practitioners’ well funded and very successful legal support team.
The HDC protection of the New Zealand Government
The following is a briefing by the office of the Health and Disability Commissioner to the incoming Minister of Health, in November 2017:

https://www.beehive.govt.nz/sites/default/files/2017-12/Health%20and%20Disability%20Commissioner.pdf
If the HDC were routinely carrying out their legislative duties, this would be their function. However, unfortunately the truth is far removed from this intended function:
- The lack of action and accountability the HDC has taken on consumer complaints would suggest that the HDC’s primary responsibility is to ensure the deception of promoting and protecting the legally enforceable rights of consumers of health and disability services is maintained.
- The lack of action taken by the HDC, in turn, is protecting the New Zealand Government from bad exposure.
- The HDC arguably, also, continue work in partnership with the ACC to protect this organisation from patient injury claims. Consistent rulings by the HDC of no breach of rights provide patients with no course of action for redress.
- Under our statutory authority, no health provider or medical practitioner is likely to ever see this inside of a courtroom for medical malpractice that has resulted in injuries and/or deaths.
By examining the evidence (see supporting links and posts on this website) it is arguable that the core objectives of the HDC are:
1. HDC task objective: Resolve Complaints
The HDC should be using their statutory powers to thoroughly investigate complaints in an open and transparent measure. Unfortunately, the HDC, as gatekeeper, has lost its consumer-centric focus and instead protects medical practitioners at all costs. Their gatekeeper protection includes:
- Ensuring a tiny number of complaints that ever undergo a formal HDC investigation ever see the light of day.
- Ensuring that costs are kept to a minimum by rejecting complaints.
- Ensuring minimal investigations will take place regarding clinical matters.
- Ensuring a negligible likelihood of complaints being upheld – finding a breach of rights. Also, a negligible likelihood of referrals to the HDC Director of Proceedings for further investigation.
- Ensuring fewer medical practitioners face disciplinary charges through the Health Practitioners Disciplinary Tribunal.
- Ensuring fewer complaints ever make it to the Human Rights Review Tribunal (HRRT). No health consumer can make a direct complaint to the Medical Council or Health Practitioners Disciplinary Tribunal or bring proceedings before the HRRT without a prerequisite HDC investigation and resulting breach finding. As a result, injured patients have brought few complaints or HRRT proceedings, and none have led to a substantive hearing.
2. HDC task objective: Improving quality and safety within the sector.
Unfortunately, under the watch of the HDC safety and quality within the health sector is deteriorating. The only verifiable reporting mechanism for the HDC – relating to improving quality and safety, actually confirms that the opposite is occurring. Health consumer complaints are increasing every year, without an end in sight. In 2017, the HDC report that complaints against DHB’s increased by 1/3rd.
The HDC claims to be uniquely placed to see trends and issues in the health sector – and to be able to raise issues with the Ministry of Health. As such, over 99% of recommendations made to health providers have been acted upon. However, after over 20 years of protecting health consumers’ rights and with an objective to improve quality and safety – why are there yearly increases in complaints? Do these means nothing? There is absolutely no verifiable evidence of how patient safety and quality of health providers has actually improved under the protecting governance of the HDC.
3. HDC Task Objective: Appropriately holding health providers to account
There is consternation about the meaning of ‘appropriately’ holding providers to account. The HDC has taken a ‘learning not lynching’ approach to incidences of medical malpractice. This softly, softly approach by the HDC ensures no medical practitioner, or health service provider will ever see the inside of a courtroom for behaviours, acts and omissions frequently causing injury and death to patients. Patients are being harmed and quite simply their legal rights are not protected. Medical fraud, malpractice and gross acts of negligence resulting in death are left unpunished. Medical professionals are getting reassurance every time the HDC refuses to take action – medical professionals are given the green light to continue to practice clinical freedom.
Examples of this are seen many times over – Doctors’ have been blatantly withholding from patients alternative treatment options and the risks of surgery for laparoscopic cholecystectomy.
The difference between the public’s ideologies of ‘holding providers to account’ verses the HDC ‘appropriately’ holding providers to account are the result of an illusionary perception. Medical fraud, gross negligence or recklessness resulting in patient harm or death (manslaughter) has simply been decriminalised by the actions of the HDC. Making an apology or conducting a self-review of practices is the only likely outcome for repeat offenders.
4. HDC task objective: Promoting the rights of consumers
It is the responsibility of the HDC to promote the rights of consumers under the Code of Rights. However, once again this function of the HDC is merely illusory. Repeatedly denying complaints and a lack of action by the HDC has the result of leaving patients and victims of medical malpractice with very few options.
“Prospect of a civil claim is an illusory form of accountability for injured patients” – New Zealand Ombudsman, health consumer legal rights.
The HDC – Tells the Government what it wants to hear ” complaints resolved”

HDC annual reports to the government always advise of great success with resolving heath consumer complaints . HDC 2017 report advised the government it had HDC resolved 2,015 complaints. Glowing results as the HDC meet it objectives which also included increased public awareness. No failure of the HDC to achieve any measurable increase in quality of health services identified?
HDC 2017 report: “Increasing volume of complaints – Over the past five years, HDC has faced ongoing and accumulative increases in the number of complaints received . Since 2012/13, there has been a 37% increase in complaints received. The 2,211 complaints received in 2016/17 represent a 13% increase on the 1,958 complaints received in 2015/16. This increase in complaints is consistent with international trends, and is due to a number of factors, including the increasing public profile of HDC, increasing awareness among consumers of their rights, the accessibility of the complaints process, and the increasing health service activity. In 2016/17, HDC resolved 2,015 complaints“
The truth, “Resolution” does according to the Act that the Commissioner works under mean anything from doing nothing, to making a recommendation (for future improvements, more training), to perhaps give a warning, or to refer a serious breach of the Code to the Director of Proceedings. In most cases though much discretion is available under the Act, and it is used in the vast majority of cases, is to do nothing at all except dismiss.
This is what health consumers had to say in a survey . Questions of this nature never again presented for feedback
Annual Report 2008-09 showed just 67 percent of complainants (then listed separately from providers) felt their complaint was taken “seriously”, only 62 percent felt the complaint had been dealt with “fairly” and only 64 percent felt their complaint had been “dealt with impartially. More alarmingly, only 57 percent of complainants understood the reasons for the decision and only 54 percent were “satisfied” with the “management” of their complaint.
The HDC – A scary reality for Health Consumers
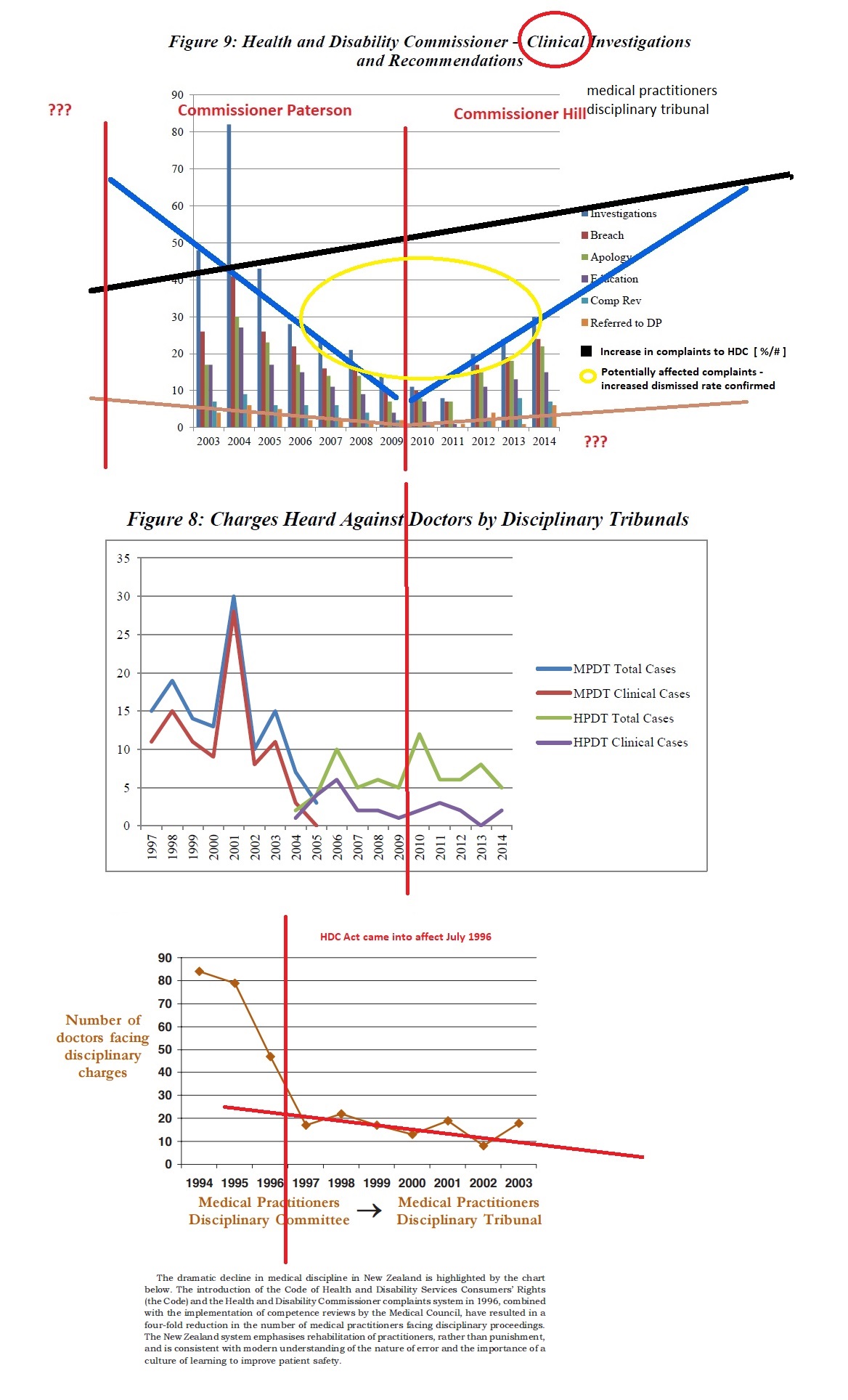
In 1996, the then Labour Government implemented the Medical Practitioners Act 1995, which provided a regulatory system – giving new powers for the Medical Council to undertake a confidential behind closed doors ‘competency review’. This was preferred to the transparent nature of disciplinary proceedings. Following implementation of the Act, medical disciplinary hearings dropped from 90 to just 3, recorded in the year ending June 2012.
Legislative reforms in the mid-2000’s saw the HDC Act further changed to protect medical service providers, the Government and medical practitioners:
Amendments made to the Act in 2003 provided the HDC with greater powers to dismiss complaints. These included requiring a preliminary assessment of a complaint followed by a decision to: –
- Refer the complaint to the appropriate regulatory body;
- Refer the complaint to the provider;
- Refer the complaint to an advocate;
- Mediation;
- At the sole discretion of the HDC, take no further action (to investigate); or
- Investigate the complaint
These changes to the Act lead to an evident decline in formal investigations, which has continued over the years and effectively decriminalised offences committed against health consumers.
Templates for resolving complaints used by the HDC includes the following:
- It seems that the care you received was in accordance with accepted standards. I accept opinion of Dr XXX… would take no further action.
Or
- It seems that your health complications post Cholecystectomy are not a result of treatment…..I accept opinions of your treating Dr XXX, (no other advice sought)….. would take no further action.
The best example of exposing this protection provided to the Government and ACC from having to provide medical support services can be found with transvaginal mesh implants victim complaints where the HDC refused to carry out an independent inquiry or uphold patent rights.
The HDC ruling example – Complaint dismissed (no breach of rights):
“It seems that the care you received was in accordance with accepted standards. I accept DR X (we are unable name this surgeon) advice that xxx procedure using synthetic mesh were appropriate operations to perform, are not experimental and have been properly researched and tested” – No further action to be taken.
Human Rights question: Will the HDC ever admit its protection policy harmed mesh recipient patients?
The answer is no – many patients overseas are suing their health providers, including the public sector. Because of ACC, New Zealand victims are blocked from taking this course of action. Also, any investigation or inquiry by the HDC would expose the Government to a risk far greater than that seen from the Cartwright Inquiry. There only has to be a look at the situation with Ethicon – a wholly-owned subsidiary of the multibillion-dollar company Johnson and Johnson, one of the main producers of mesh implants and currently the subject of multiple class-action lawsuits for causing harm to health consumers.
(See case study below on the treatment of mesh patients in New Zealand)
Further changes to the Act, in 2009, saw the final nail in the coffin for consumer rights. The HDC took action to cripple the effectiveness of health advocacy services and the legislative requirement for its independence to support health consumer rights. A restructure of public health advocacy services took place, based on the recommendation of the HDC to the Government. This HDC recommendation rejects the advice of The Director of Advocacy, New Zealand Council of Christian Social Services and the New Zealand Federation of Business and Professional Women Inc., which recommended options that would retain the greater independence of advocates and the Commissioner‘s impartiality.
Central points raised by the HDC, in 2009:
- Advocates are not impartial but take the side of the consumer
- Advocate staff hiring and position descriptions are not under direct HDC control
As a result, changes to Health Advocacy Services staff were brought under the payroll and control of the HDC. This is in direct conflict to key recommendations of the Cartwright Report:
- “For advocates, who would be on the side of the consumer, to ensure that consumers’ rights were upheld”
Position description objectives were watered down to no longer reflect the key recommendations of the Cartwright inquiry and ensure that consumers’ rights were upheld, but were replaced with new objectives of Health and Disability Advocates – ‘learning from experiences’. Advocacy staff with legal skills have become noticeably absent. Currently, advocacy staff of the HDC refuse to assist with victims who have had treatment support for treatment complications withheld – and they will not offer consumers legal advice.
Two other recommendations to Health and Disability Commissioner Act 1994 that cemented greater HDC control on Health consumer rights made also were:
1] The infamous ’you lie for us, we protect you, you are protected‘ policy made to external advisers by the HDC. I.e. MESH surgeons opinions provided, ’Synthetic MESH is safe and tested’ or with cholecystectomy patients ‘ Experience of the surgical community over the last hundred years is that digestive tract complication side effects are not an effect of removing the gallbladder function’ etc., opinions also used to block patient access for support through ACC as according the HDC ‘no breach of rights taken place, no injury has occurred’.
- “Provide expert advisors contracted by HDC with the same degree of immunity enjoyed by employees under the Crown Entities Act”
2] greater powers for the HDC ‘guard dog control’ or block heath consumer complaints being investigate by appropriated and independent authorities.
- “Clarify that the Director of Proceedings may take action only upon referral from the Commissioner”
https://www.hdc.org.nz/your-rights/about-the-code/review-of-the-act-and-code-2009/
How the HDC protects the Ministry of Health from being held accountable – A Case Study on synthetic mesh implant victims
The HDC can be held directly responsible for a lot of suffering of mesh victims for omitting to hold medical practitioners to account.
Compare the HDC response to the safety of health consumer recipients of mesh products in New Zealand to that of other countries. The response from New Zealand and the HDC was that they are ‘monitoring complaints’ Conversely, the response from Australia and the UK resulted in Australian Senate / Royal Inquiries and involved contacting all affected patients to gather full information about the extent of the issue.
In 2016, a Government Health Committee asked why didn’t the HDC carry out an inquiry into the effects of surgical mesh? The HDC response was simply that they acknowledged that surgical mesh is an important issue, and that it takes it very seriously.
The HDC took the following action:
- In 2011, the HDC met with the two petitioners and some other advocates.
- In 2016, the HDC wrote to providers and the regulators asking how the ‘relevant colleges’ were managing the current ‘knowledge’ about mesh and its risks, and asked how patients’ were informed about the risks of surgical mesh. Assurances were received so the HDC did not consider that an inquiry was necessary, but it continued to ‘monitor any complaints’.
- The HDC took no action but will monitor complaints
https://www.parliament.nz/resource/en-NZ/SCR_72706/f9291c110b22ab02cdcb7d937df63f5a6259673b
The truth is that many New Zealand patients were harmed and some left with crippling heath complications and no treatment support, and blocked from access to ACC because the HDC asked the wrong questions. They should have been prioritising patient safety under the Code of Rights. The HDC should have asked: Are mesh products harming patients? Is patient safety at risk?
The HDC claimed they were protecting consumers, and advised that the HDC:
- Are increasing the focus on consumers with more transparency,
And reducing the incidence of preventable physical injury and death
An HDC ruling provides evidence to the contrary. A patient was blocked from treatment support by an HDC ruling. HDC “It seems that the care you received was in accordance with accepted standards. I accept DR x advice that laparoscopic sacrocolpopexy with synthetic mesh and TVT sling surgery (using mesh) were appropriate operations to perform, are not experimental and have been properly researched and tested” No further action to be taken, and no investigation whatsoever – the complaint was simply whitewashed.
This ruling was made at the very same time these mesh procedures were suspended in Scotland and many countries went on to follow suite, including New Zealand. The reasons for this was that the mesh products were ‘never property tested or certified fit for human use’. The HDC have no justifiable evidence to support their original position. Their ruling simply had the effect of blocking victims of this treatment from receiving post care support. The HDC hold firm that no injuries took place from faulty defective products. Many New Zealand women were left to suffer inhumanely, as a result of this decision (and many other protection rulings), by the HDC.
Several years later the product was banned across many countries. A few years later, the UK watchdog released this:
2018: Justice at last? Health officials to investigate scandal-hit vaginal mesh implants that have left thousands of women ‘suicidal’
The Department of Health and Social Care will begin conducting a national audit. This will tell them exactly how many women the devices have affected. Thousands of victims claim to have been left on the brink of suicide from mesh.
“Government to review thousands of harmful vaginal mesh implants” https://www.independent.co.uk/news/health/vaginal-mesh-implants-audit-government-thousands-women-health-risk-scandal-latest-a8185061.html
Today, the HDC still refuses to acknowledge patient safety risks for mesh recipients. Australia, on the other hand:
“TRANSVAGINAL MESH. 22 November, 2016 – Senator Derryn Hinch addresses the Senate https://www.youtube.com/watch?v=itoGIBAX0FY “Suicide is the only option” for these victims to end the pain and transvaginal or pelvic mesh linked to “greatest medical scandal of mothers in Australians history”
The transcript can be found here https://www.openaustralia.org.au/senate/?id=2016-11-22.142.1, which also details more feedback confirming the New Zealand position and the HDC deception presented to the New Zealand Government and covering up the breach of Right 4(1) of the Code of Rights, the right to have services provided in a manner that minimises the potential harm to patients, and optimises quality of life.
As noted several times, one of the key roles of the HDC is to protect patient safety. The Commissioner does not have to wait until a complaint is received before they can take action. The Act enables the Commissioner to undertake investigations on their own initiative. Commissioner Hill advised this is one of the ways the Commissioner fulfils his role of “consumer watchdog”.
Feedback on HDC from mesh sufferers:
“Rose Wall (deputy commissioner) told us last October (2016) that they had received ‘very few’ complaints about mesh, particularly about lack of informed consent“
“HDC were a total waste of a year of my life, and took the implanting surgeon’s word over mine. I thought they were there for the patient.”
“I haven’t bothered with them (making a complaint with the HDC). After a very long phone call, the woman on the end of the phone simply told me they were “aware of many complaints”. I said that as I’m so sick I could do without the stress. I told her that DR x had, in actual fact, put a totally different sling in to what he said.. plastic instead of porcine” (He destroyed my notes… but as I request copies of all my letters. I still have my copies) My new UG did a biopsy last December and it is definitely plastic. My rheumy says that while we cannot prove the plastic caused my immune diseases, my body is in constant state of inflammation, which has made me very ill. ”
“It just takes energy and when you are suffering from all the mesh complications it’s hard to put in all in writing (HDC complaint). They (the HDC) will protect the surgeon I found“
“They (the HDC) did with me, and that was a year long drawn out thing and then the lawyers got together and I got a crap letter, with so many inconsistencies, but they took the side of the surgeon and (HDC) told him to make sure he gave better informed consent… that idiot is still injuring women every day and hasn’t learnt anything. The HDC should be there for the patient – not be decided amongst HDC and hospital lawyers”
“Absolutely disgusted with the HDC. I know I knew it would be ineffectual, but they have basically told me that I lied. That the retention (even though I had two separate tests in two separate cities with two totally separate set of specialists proving it), the pain – the cutting feeling like something is pulling up in my abdominal and cutting through me, my inability to lie on my tummy as it make that pressured feeling harder – having to buy undies to above the hips because anything around my bellybutton level of waistline gave me horrible cramps – severe cutting feeling – sharp pain when urinating – feeling absolutely exhausted all the time, was all in my head!”
“They (HDC) trust the Gynes here that blatantly lied, lied about berating me when I said the surgery went wrong, told me that I had ghost pain, told me no scan was possible to see where the mesh was in my body. They were all about ass covering. The HDC trusts them more than me, or the surgeon who actually managed to remove the mesh (or most of it). The fact that I no longer have those symptoms (retention, pain, extreme fatigue, constant infections – still have some but not constantly) means nothing. A part of me thinks I should not have complained (made a complaint to the HDC). I expected them (HDC) to be ineffectual. I did not expect them to be down right insulting. To tell me that it was in my head”
“I find the New Zealand medical system for complaints procedures toxic. The specialists I have come across have been grossly unprofessional, and colleagues back each other up. There are no consequences. The HDC takes the word of the people you are complaining about rendering it totally ineffectual.
“They should be consulting independent specialists overseas”
“The HDC favours the surgeons as they have lawyers that just argue it out and after 6 pages of lying and an inconsistent story from the implanting surgeon it was basically what the surgeon says is right… Who would want to make up what has happened to us. They are corrupt and a waste of the taxpayers money. I wouldn’t even bother to complain to them again as it a waste of energy that I just don’t have”
“I was told ACC do not police the Doctors – HDC would do that? But in a lot of cases HDC turn us down before they even start to look into the incident”
“I applied for my HDC file: under the Privacy Act: A bombshell. Writing to me saying they were investigating case. And the very same day their ‘internal’ meeting- said, “do not investigate complaint”. HDC lying to me about my valid complaint. They are disgusting. And my ACC case manager then moved to HDC and became my HDC case manager.”
“Anyone else receive a condescending letter from the minister of health today?”
This letter was received from the new Labour Minister of Health:

- The use of surgical mesh implants is possibly one of the greatest medical scandals and abuses in medical history, and is arguably worse than thalidomide. The existence of health completions as the result of surgical mesh has left patients suicidal and in constant pain. Patients have suffered horrible panful deaths from side effects – described as torture.

- The issue with this statement is that health care professionals have been withholding the truth about product defect risks and alternative treatment options Thousands of lawsuits worldwide are in progress as the result of the use of mesh products. Arguably, surgeons are not disclosing the risks because they believe they have the clinical freedom to treat patients as they see fit, under the protection of the HDC.
- The Health Minister is refusing to acknowledge the following:
- Mesh is killing patients.
- The HDC is covering up the scandal by dismissing complaints
- Patients have been critically injured and many left disabled without any course of redress for treatment or support.

- The Minister arguably has knowledge that the complaints process through the HDC is ineffectual. The HDC is in fact operating as a protection service for the Government and ACC rather than its intended statutory function as protector of consumer rights.

- Patients have contacted Medsafe several times over several years. The use of mesh has been suspended by many countries through regulations. In this instance, the patient is told to log a new issue with Medsafe.
- ‘There is no pre-market approval process by Medsafe. Our New Zealand legislation (Medicines Act 1981) does not require medical devices to go through an approval process before being placed on the market. Medsafe does not, therefore, assess information about a device, review clinical trials, review documentation and warnings or perform any other assessment before a medical device can be used. The only requirement is that the sponsor (usually the manufacturer or the importer) must list it electronically on a database (operated by Medsafe) within 30 days of it being first supplied (this is not an assessment or approval). This is unlike other parts of the world (Australia, North America and Europe) where some form of assessment is required before certain medical devices can be marketed.’
See – Surgical Mesh in New Zealand Submission prepared for The New Zealand Health Select Committee by Carmel Berry and Charlotte Korte 14 May, 2014
Successful complaint exposes the extent of the fraud:
A lone testimony of a patient who fought against the HDC and won, exposes the protection established in New Zealand to block patients’ rights, right to quality life and the cover-up of patient harm.
The HDC, ACC, Medsafe and Fairway Mediation, all Government bodies, are arguably joined at the hip with one specific objective – to protect each other at the expense of patients.
“I have made a complaint to Medsafe ages ago and I think from memory, my Doctor did one too, except the Surgeon, who sides with ACC was adamant my case should remain “as no injury found”. But knew it was the mesh! I have complained to ACC all the way, at every rejection.
I have already written to the previous Minister of Health, and the new Minister of Health – three times. And the same to our Prime Minister – three times.
And finally, I got replies and an investigation into ACC practices, as it was costing them more in trying to stop me getting surgery. I even had a lawyer on the job, “who works for us” and becomes our advocate too. I have exposed Fairway Mediation- it is Government owned now and has to be biased in my opinion, from the investigations I have done. I have finally won my case and as my Doctor thinks and says, “this will open the floodgates”. I believe we are already getting more and more cases heard and won.
The last step, if not won, was to go to the HDC, but as I won my case I cannot see the need, as they would say I have been attended too and the hernia mesh has been taken out. I would not look good complaining to them, when I have had my rights restored. My only complaint to them would now be the time it took to fix the injury they did to me, and not informing me of the placement of the mesh or any adverse effects etc.
No one is the same after having mesh put in!”
Compare the above New Zealand Government response to an Australian call for a senate investigation, which did take place:
TRANSVAGINAL MESH. 22 November, 2016 – Senator Derryn Hinch addresses the Australian Senate demanding an enquiry take place and goes on to state some more facts:
https://www.youtube.com/watch?v=itoGIBAX0FY
The mesh is meant to be chemically inert. Before any implanted device gets clearance from the FDA in the United States, there must be guarantees that it is inert, that it is safe, that it will not change after contact with tissue fluids in the body, that it will not produce allergic reactions, that it will not incite inflammation, will not trigger your immune system, will not harbour and breed bacteria and, crucially, will stay anchored wherever in the body it is installed. Tragically, painfully, dangerously, transvaginal mesh does not pass any of those tests.
- These mesh hammocks are anchored deep into thigh and buttocks and pelvic region muscles where nerves grow through the mesh, making it almost impossible to remove when things go wrong. It is like a wire-netting fence getting overgrown with creeper in the backyard.
- This ‘harmless’ plastic netting can become brittle and start to break away in shards and splinters. They start to float around the body, causing inflammation and excruciating pain. These slings have been called ‘a torture device’. There have been cases where a splinter has pierced a woman’s vaginal wall and injured her partner during intercourse. No wonder some of these mesh products have been totally banned in Scotland. No wonder there are nearly 100,000 lawsuits pending in the United States over mesh.
This investigation goes on to quote more alarming testimonies. In New Zealand, according to the HDC, patients are not at risk. The HDC claim it has received assurances from undisclosed authoritative sources.
As in New Zealand, “some Australian women have taken out loans, or increased their mortgage, to cover the $35,000 to $40,000 costs of having the operation overseas…suffered interminable pain, and physical and mental breakdowns, because of these mesh implants. Some have thought that suicide was the only solution, having first been told that there is only a one per cent chance of an adverse reaction. They have since been treated like mushrooms: kept in the dark and fed bullshit by doctors, hospital administrators, the drug companies and even the TGA. At times they have almost believed that their pain and their debilitative condition was psychosomatic or just a way to get more painkillers. That is what the doctors suggested”
Senator Derryn Hinch goes on to ask the fundamental question: Why would the TGA (equivalent to New Zealand’s Medsafe and the HDC) allow this, or why would surgeons’ implant a permanent and life-threatening device into a woman’s body for a non-life-threatening problem such as incontinence or prolapse? The foreign body mesh placed through the vagina cannot be safely removed and it is guaranteed to pick up bacteria, which will fester and create a biofilm. Erosion, nerve and organ damage can happen immediately or, as we have seen, it can take up to 15 years.
“None of us were warned that removal of mesh is pretty much impossible. Few of us were warned that it was polypropylene. None of us were warned that there were over 100,000 legal cases against mesh and warnings throughout the world. None of us were tested for allergy or reaction to polypropylene before surgery. Most of us were only offered mesh as a treatment.”
“I read these women’s case histories and also read about some of the other side effects: infection, bleeding, painful sexual intercourse, vaginal scarring, prolapse return, sepsis, immune system rejection, urinary problems and chronic pain. It just goes on and on and on. It is a national disgrace. Just think of the pain of daily living: stabbing pain when sitting on the toilet, stabbing pain when crawling into bed, pain when walking, pain when sitting at a desk.”
Senate debates, Tuesday, 22 November 2016, Transvaginal Mesh https://www.openaustralia.org.au/senate/?id=2016-11-22.142.1
Additional background reading on the HDC
Fewer charges are being laid in the health practitioner’s disciplinary tribunal: Should we be concerned? Victoria University of Wellington Law Review, Volume 46 Issue 4 (Dec 2015) Davies, Kim
http://www.nzlii.org/nz/journals/VUWLawRw/2015/49.pdf
Pictures speaking volumes